Challenge
A large pharmaceutical company had a novel cancer immunotherapy approved by the FDA for late-stage lung cancer. Field Medical Teams were mobilized to engage with oncologists at major US health systems and payers. The field teams discovered that despite strong clinical evidence, key health systems and payers established or followed standardized treatment pathways (e.g., National Comprehensive Cancer Network (NCCN) pathways), creating barriers to using the new therapy. As a result, patients were at risk of being uninformed of this effective treatment option, making them less likely to discuss it with their oncologist. There was also a risk of the immunotherapy not reaching the sub-population for which it had proven to be efficacious.
Approach
Creating an Effective Rapid Response Team
Point B helped our customer form a cross-functional strategy team to address access challenges and biosimilar competition. The new pilot team included cross-functional representation from Medical Affairs, Commercial, Legal, and Regulatory Affairs.
After Point B identified the strategy team, we partnered with our customer to establish governance structures, facilitate planning and objective workshops, and drive cross-functional compliance.
The team was tasked to complete a rapid assessment of the treatment landscape to better understand how health system treatment protocols impacted therapy use. From there, they developed evidence-driven strategies to overcome those barriers. Point B led the strategy team to identify and understand the root causes, including:
- Analyzing patient access impact across multiple health systems, payers, and cancer organizations’ treatment pathways
- Assessing treatment pathway adherence to clinical studies and real-world evidence for comparable cancer therapies
- Developing appropriate responses to remove patient barriers by communicating evidence-based clinical efficacy and cost-effectiveness to oncologists and payers
Operationalizing the Strategy Team
The strategy team's insights needed to be integrated into the field team’s operating model to make the information actionable and impactful. Point B created processes and capabilities to operationalize the strategy team findings.
Barriers like current treatment use, standard of care, and customer awareness helped inform the strategy team’s work with enablers like analytics and visualization tools in working with academic research centers to create Real World Evidence (RWE). Evidence analysis and insights were an internal function of the strategy team. This capability allowed the team to aggregate and analyze current dynamics and data to extract evidence gaps. Partnerships with leading academic institutions were created to bolster research collaboration for any net-new RWE needs. The strategy team managed new supporting evidence and then used it to equip the field team with new information to share with customers. As a result, the field team was better informed about combating barriers while educating oncologists and payers on the evidence-driven benefits of the new therapy.
This operating model created a continuous RWE feedback loop that increased product education and, ultimately, market share.
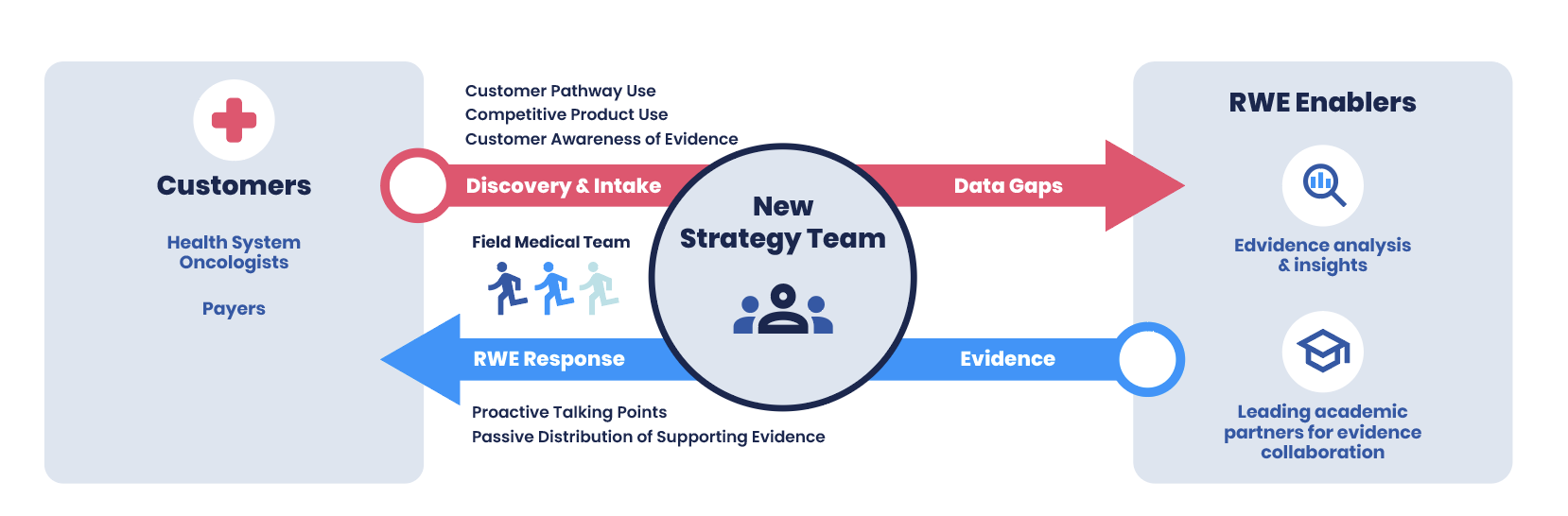 Figure: Overview of Process to Uncover and Address Treatment Pathway Obstacles
Figure: Overview of Process to Uncover and Address Treatment Pathway Obstacles - Discovery and Intake processes to gather pathway information, discover prescribing challenges and pathway adherence, competitor therapies used, and oncologists’ awareness of the latest evidence.
- Evidence Analysis and Insights to assess whether pathway-driven decisions conformed to published clinical and real-world evidence, and support creation of evidence-driven responses. As intake volume and diversity increased, Point B created data analytics and visualizations to better categorize insights by oncologists' awareness of evidence, cost of care, rigidity of pathways, and other factors.
- Appropriate Responses: The strategy team crafts “proactive” and “passive” response strategies. Proactive responses included approved talking points and published evidence for Medical Science Liaisons, study presentations at upcoming conferences, etc. The medical affairs team crafts passive responses by performing meta-analysis of existing evidence shared with the oncologists and hospital networks via publications and other channels.
- Research Collaborations to Increase RWE: To generate additional evidence, Point B managed the formation of a research initiative at a leading academic institution. The initiative focused on patient-centered approaches to treatment options resulting from greater patient awareness and agency in jointly choosing appropriate cancer treatments, including clinical trials.
Project Outcomes
Strong results demonstrated the need for a permanent, scaled strategy team.
The approach demonstrated the ability to influence pathway updates – from off-pathway to on-pathway or from on-pathway to preferred-pathway in specific therapeutic areas, cancer stages, and sub-populations. Early success supported our customer’s decision to scale the strategy team. The capability to address an increased volume and complexity of pathway issues with evidence needs and responses is incredibly valuable to commercial teams. As part of this expansion, Point B developed analytic and visualization techniques to improve the categorization and analysis of access issues, giving executives better insight into barriers, solutions, and, ultimately, outcomes.
If you're interested in speaking with one of our life sciences experts about medical affairs, RWE, or building capabilities to address adoption barriers, please contact us today!
RELATED INDUSTRIES
RELATED SOLUTIONS